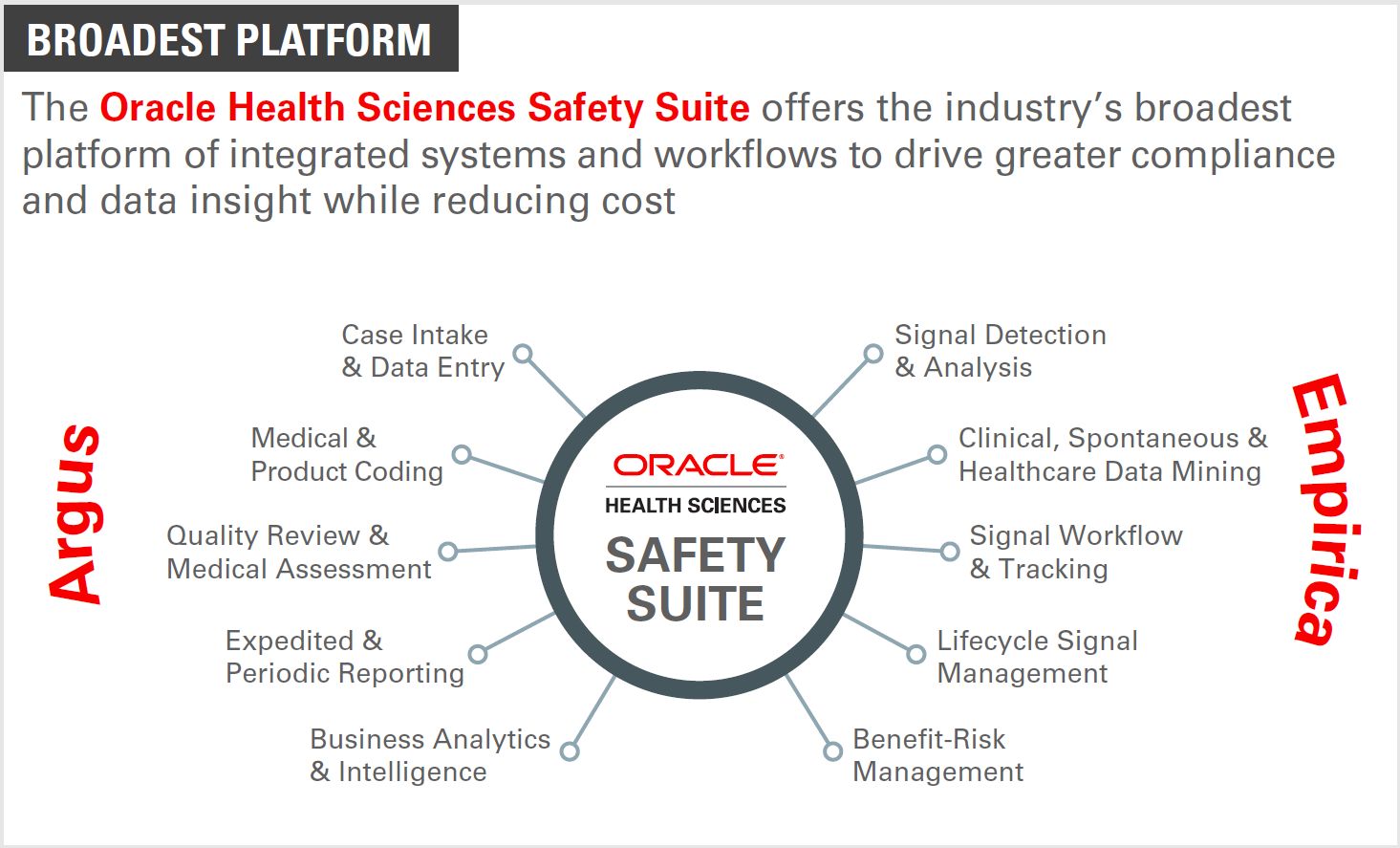
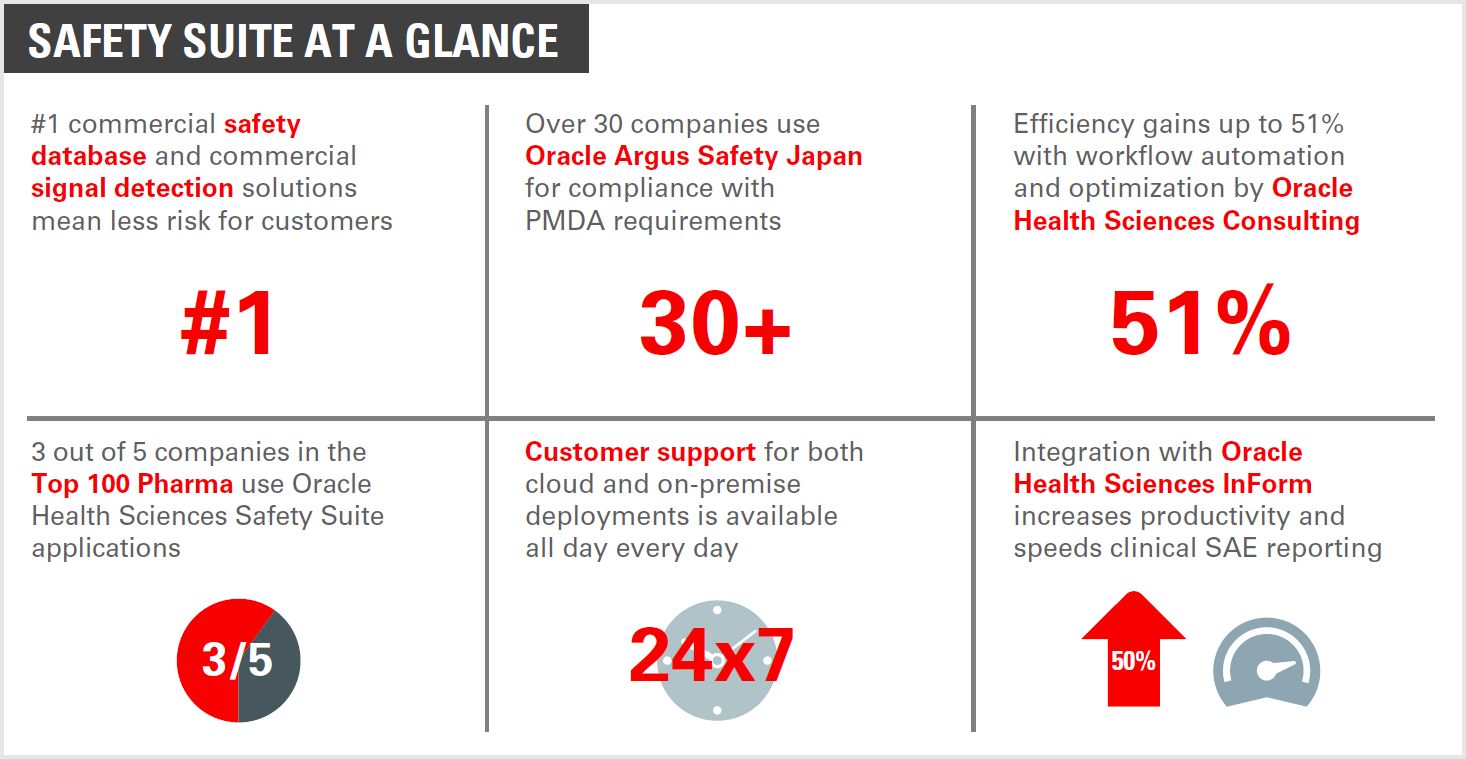
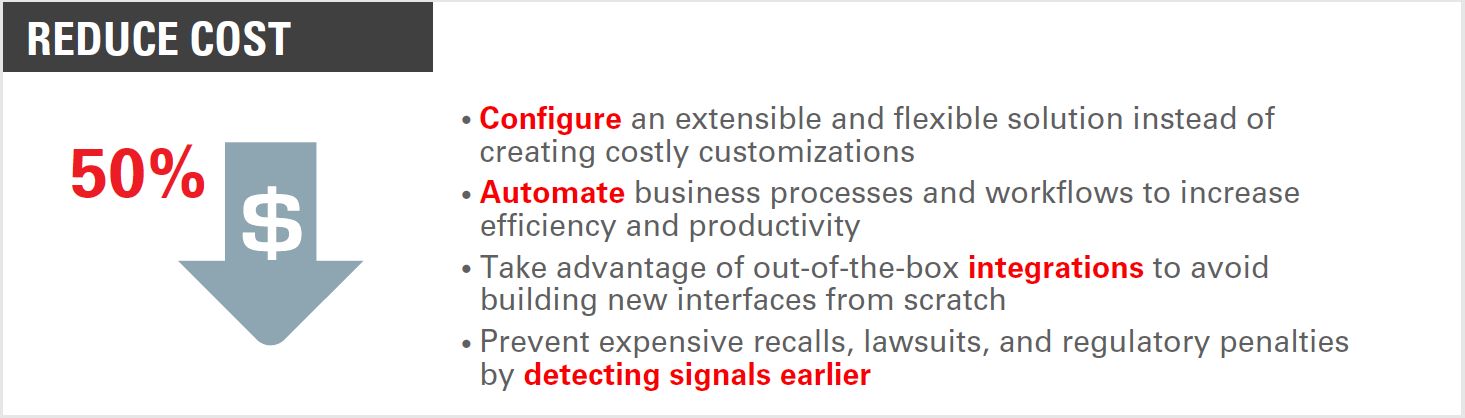
Pharmaco – Vigilance
PHARMACO – VIGILANCE


ADDRESS
INDIA
ZenRise Clinical Research Pvt Ltd,
Plot # 201, MythriNagar, Madinaguda,
Miyapur, Hyderabad, India, 500049.
Plot # 201, MythriNagar, Madinaguda,
Miyapur, Hyderabad, India, 500049.
USA
ZenRise Clinical Research,
5000 Centregreen Way # 500,
Cary, NC 27513 USA
5000 Centregreen Way # 500,
Cary, NC 27513 USA
LOCATIONS
INDIA & USA